In April 2023, the European Commission adopted a comprehensive package of proposed reforms to the legislation governing the authorisation of medicines in the EU.
One of the most significant and widely criticised reforms was the proposal to reduce the minimum period of data protection for new medicines approved in the EU, while introducing mechanisms to offset this reduction.
In March 2024, the European Parliament proposed reforms that were less drastic than the original proposals from the European Commission. However, these reforms still incorporate many additional incentives that surpass those provided by the current system.
In June 2025, the European Council established its own position on these proposals ahead of negotiations with the European Parliament, as will be discussed below.
Negotiations between the Commission, the Parliament and the Council have now commenced. Whilst certainty is still some time away, we anticipate an update on the final form towards the end of 2025 / early 2026.
In the meantime, it is encouraging for innovators that the amendments to the minimum period of data protection proposed by both Parliament and Council are more generous than the Commission’s initial proposals. For now, the existing regulatory exclusivity regime will remain in place, as it will for all marketing authorisation (MA) applications for reference medicinal products submitted before the new legislation takes effect.
Standard regulatory data & market protection
Current regime
Under the current system, new medicinal products benefit from the EU’s “8+2+1” regime.
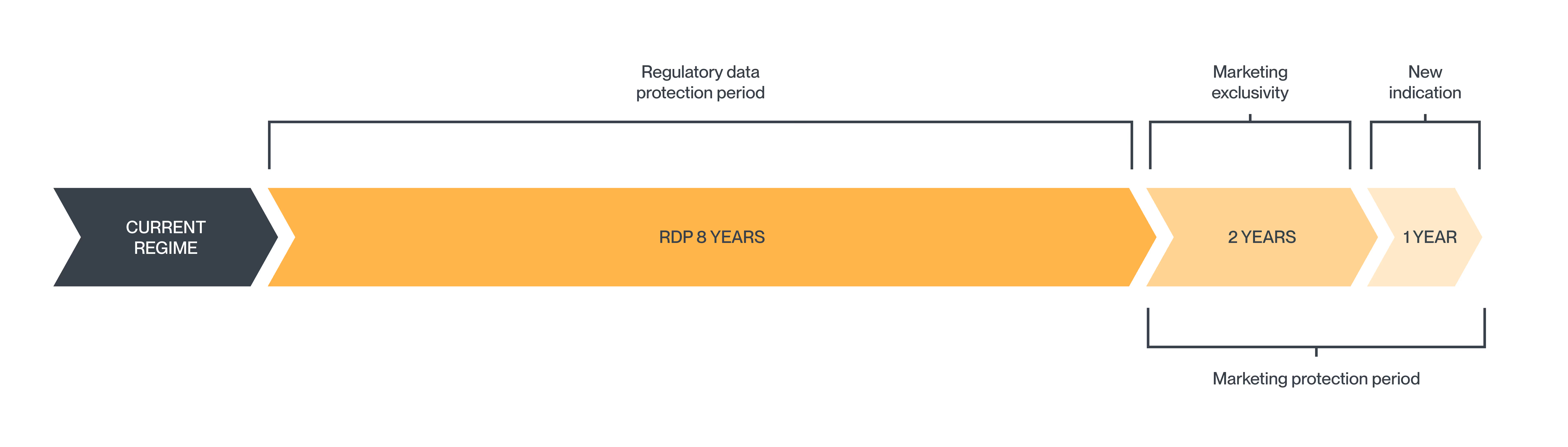
- 8 years of data protection, during which the marketing authorisation holder has exclusive rights to the clinical and preclinical data for the approved product.
- 2 years of market exclusivity (ME), during which third parties may use the data to support an application for their competitor products and such applications may be approved, but the product may not be placed on the market.
- 1 year of additional market exclusivity, if, during the first 8 years of approval, the MA holder obtains authorisation for at least one additional indication which provides a significant clinical benefit compared to existing treatments for the new indication.
European Parliament’s proposed reforms
The European Commission initially proposed shortening the minimum period of data protection for new medicines approved in the EU from 8 years to 6 years. However, the reforms proposed by the European Parliament would reduce this period from 8 years to only 7.5 years. The current additional 2-year market exclusivity period remains unchanged.
In its proposed reforms, the European Commission announced additional incentives enabling an MA holder to extend the data protection period to a maximum of 10 years. While many of these additional data protection periods remain in the reforms proposed by the European Parliament, a cap has been introduced limiting the maximum data protection to 8.5 years.
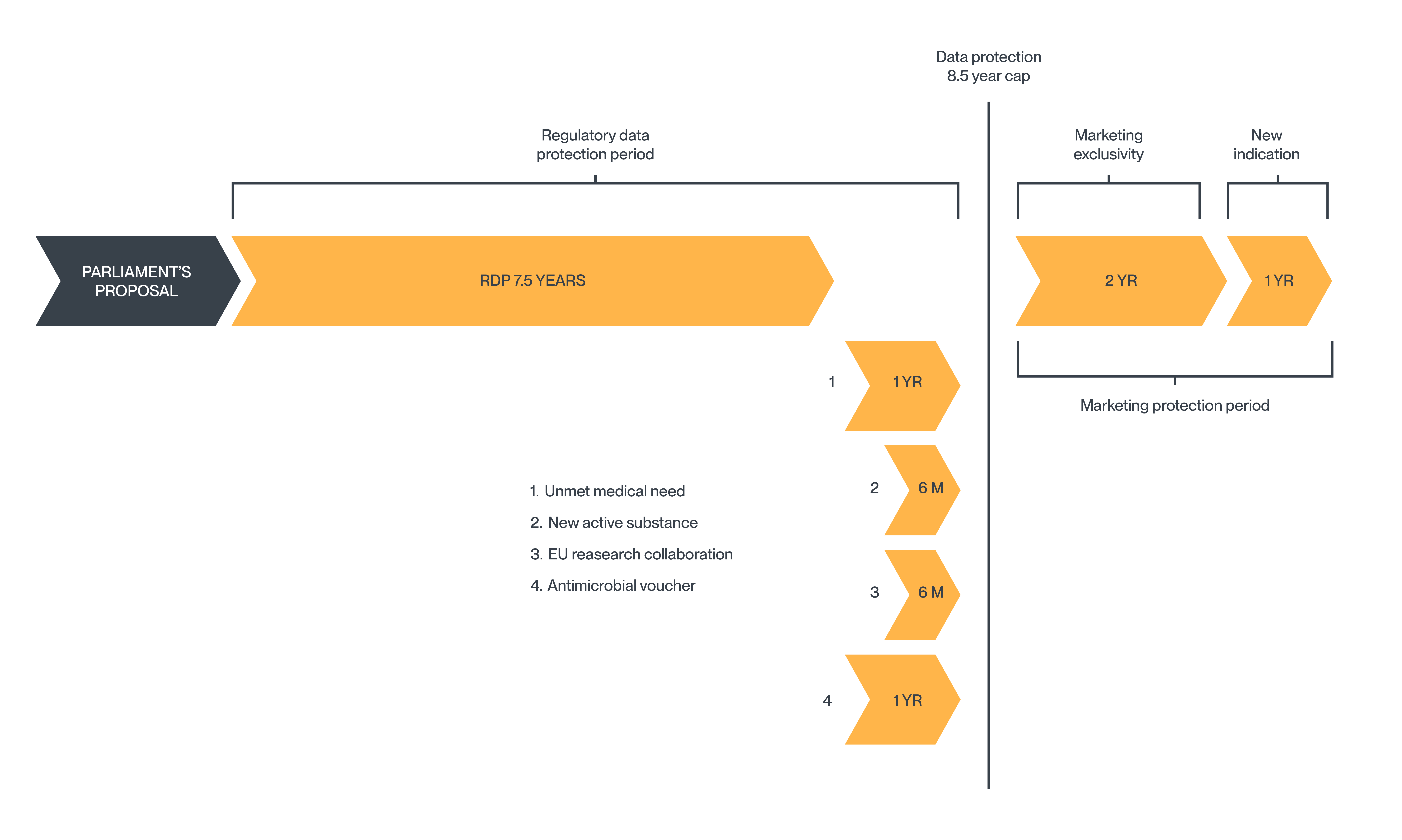
Additional incentives
- 1 year of additional data protection for products relating to a drug that meets an unmet medical need (increased from 6 months in the Commission’s original proposal).
- 6 months of additional data protection for new active substances that have undergone comparative trials against the best-known drug for the treatment of the disease.
- 6 months of additional data protection if a significant share of the product’s research and development takes place in the EU and at least partly in collaboration with EU research entities. This replaces the Commission’s original proposal to provide 2 years of data protection for continuous supply in all relevant EU member states.
- Up to 1 year of additional data protection by way of a "transferable data exclusivity voucher" awarded for priority antimicrobials. The length of the data protection extension (either 6, 9, or 12 months) will be determined by the priority level of the target pathogen. The voucher cannot be used for a product that has already benefited from the capped maximum regulatory data protection period of 8.5 years and can only be transferred once to another marketing authorisation holder.
In the reforms proposed by the European Parliament, the option to obtain a one-off additional year of market exclusivity for a new therapeutic indication that offers significant clinical benefits compared to existing therapies remains unchanged from the current regime. The European Commission's original proposal to replace this with an additional year of data protection was not adopted.
European Council’s proposed reforms
The European Council has proposed that the minimum data protection period remains at 8 years, which is aligned with the current regime. However, the Council has proposed reducing the period of marketing protection to only 1 year (down from 2 years in the current regime).
The European Council has also announced additional incentives enabling an MA holder to extend the market protection period to a maximum of 3 years. However, importantly, the Council has introduced a mechanism to revoke market protection if the marketing authorisation holder fails to meet access and supply obligations.
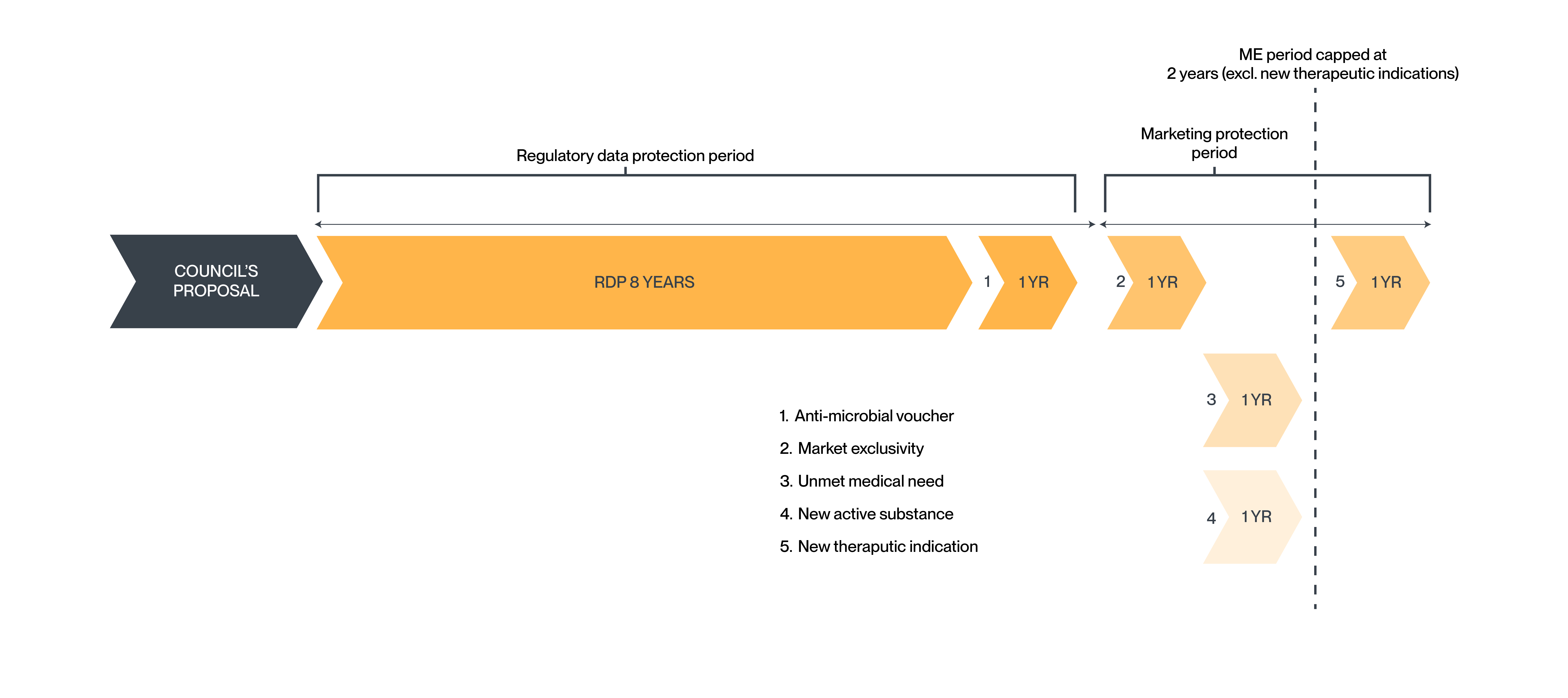
Additional proposals
- 1 year of market protection has been proposed for medicines meeting an “unmet need”. This provision was initially proposed by the Commission as a 6-month extension to the RDP period, rather than ME.
- 1 year of market protection or for new active substances that have undergone comparative trials against the best-known drug for the treatment of the disease. This provision was initially proposed by the Commission as a 6-month extension to the RDP period, rather than ME.
- 1 year of market protection if, during the initial RDP period, the product gains approval for new indications with significant clinical benefit.
- 1 year data protection by way of a "transferable data exclusivity voucher" awarded for priority antimicrobials. However, an additional requirement has been introduced in that, to obtain the voucher, the MA application for the priority antimicrobial must be submitted first in the EU, or no later than 90 days after the first MA submission outside the EU. In addition, if the voucher is used on a medicinal product other than the priority antimicrobial concerned, the voucher can take place only in the fifth year of the RDP period and if the annual gross sales of that medicinal products in the EU during any of the preceding four years have not exceeded 490 million euros.
The Council has proposed that these changes to regulatory exclusivity would take effect 36 months after entry into force of the new legislation (rather than 18 months after entry, as proposed by the Commission).
Summary of proposed regulatory protection reforms
Final thoughts
The final form of the legislation remains undecided. Nevertheless, the proposals represent significant changes to regulatory exclusivities in Europe that could have a considerable impact on the exclusivity periods for new drugs. It will therefore be necessary for innovators to keep abreast of potential changes and review their exclusivity strategies, including patent protection, SPCs and the regulatory exclusivities as the situation develops.




















.avif)


%203.jpg)

%20provider.jpg)
.jpg)
%20(1).jpg)


